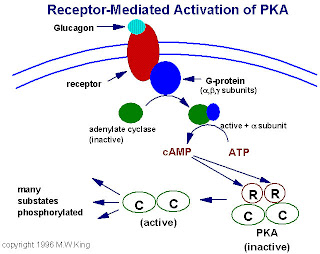
In inactive state, PKA consists of two catalytic & two regulatory subunits ‘C2 R2’ with regulatory units blocking catalytic centers of catalytic units.The binding of cAMP to the regulatory subunit (2 on each regulatory subunits) alters their conformation, causing them to dissociate from the complex.The released catalytic subunits are thereby activated to phosphorylate specific substrate protein molecules.
TURN ON
Initially the G-protein a subunit has bound GDP, and the a, b, & y subunits are complexed together.
- Hormone binding to a 7-helix receptor (GPCR) causes a conformational change in the receptor that is transmitted to the G-protein. The nucleotide-binding site on G-a becomes more accessible to the cytosol. G-a releases GDP and binds GTP. (GDP-GTP exchange)
- Substitution of GTP for GDP causes another conformational change in G-a. Ga-GTP dissociates from the inhibitory b,y subunit complex, and can now bind to and activate Adenylyl Cyclase.
- Adenylyl Cyclase, activated by the stimulatory Ga-GTP, catalyzes synthesis of cAMP.
- Protein Kinase A (cAMP-Dependent Protein Kinase) catalyzes phosphorylation of various cellular proteins, altering their activity.
TURN OFF
- Ga hydrolyzes GTP to GDP + Pi (GTPase). The presence of GDP on Ga causes it to rebind to the inhibitory b,y complex. Adenylate cyclase is no longer activated.
- Phosphodiesterase catalyzes hydrolysis of cAMP to AMP.
- Receptor desensitization occurs. This process varies with the hormone. Some receptors are phosphorylated via specific receptor kinases. The phosphorylated receptor may then bind to a protein arrestin, that promotes removal of the receptor from the membrane by clathrin-mediated endocytosis.
- Protein Phosphatase catalyzes removal by hydrolysis of phosphates that were attached to proteins via Protein Kinase A.
1 comment:
basant here
"mathi prakashit gariyeko laghukatha malai athyadhik man pareko hunale, agami din haruma pani yesto katha haru thuprai sunna payela bhanne asha ka sath mero pratikriya yehi samapta gardacho!!"
dhanyabad
Post a Comment